Test Code 22C3 Programmed Death-Ligand 1 PD-L1 22C3 Semi-Quantitative Immunohistochemistry Manual Advisory Information For information on selection of programmed cell death 1-ligand 1 PD-L1 testing see PD-L1 by Immunohistochemistry. Dako PD-L1 IHC 22C3 pharmDx.
PD-L1 IHC with Interpretation - This PD-L1 qualitative assay is intended for use in the detection of PD-L1 expression in cancers.
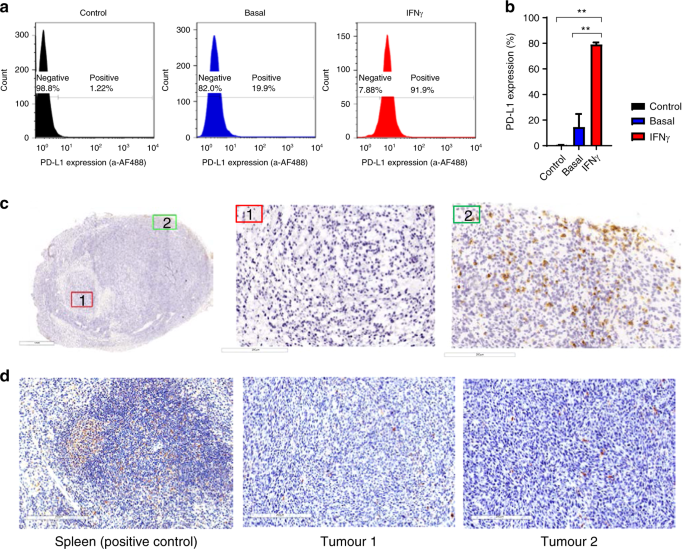
Pd l1 testing cpt code. Programmed cell death 1-ligand 1 PD-L1 also known as B7 homolog 1 B7-H1 or CD274 is a transmembrane protein involved in the regulation of cell-mediated immune responses through interaction with the receptor programmed death protein-1 PD-1. PD-L1 IHC 22C3 is indicated as an aid in identifying NSCLC patients for treatment with KEYTRUDA pembrolizumab. Please see the table below of PD-L1 IHC FDA-Approved Companion Diagnostics for more information.
By Alyssa Frank September 15 2019. Scoring and clone utilization for PD-L1 testing is based on FDA-approved indications. Clone 28-8 88360 G0452 3-5 Tissue PD-L1 Expression by.
The specific PD-L1 clone scoring method and eligibility requirements are dependent on the tumor type stage of malignancy previous. For non-FDA approved test indications or sample types order 94007-PD-L1 IHC with Interpretation. The different PD-L1 tests offered at Quest appear below.
Immunotherapies targeting the PD-1PD-L1 pathway have been reported to have durable anti-tumor effects in a. For urothelial bladder carcinoma order 94047-PD-L1 Bladder. Aetna considers testing to detect PD-L1 expression eg PD-L1 IHC 22C3 pharmDx medically necessary for persons with non-small cell lung cancer being considered for treatment with pembrolilzumab Keytruda.
Companion diagnostic testing to aid in the prediction of response to pembrolizumab KEYTRUDA as first- or second-line monotherapy for patients with NSCLC. PD-L1 22C3 IHC with Combined Positive Score CPS Interpretation pembrolizumab KEYTRUDA. PD-L1 Client Case or Reference Number.
Lung Lung Cancer Panel EGFR KRAS ALK ROS1 and PD -L1 94608 88360 88271 x2 88274 81235 81275 81276. However the result that is rendered is qualitative and the package insert from Dako indicates that this is a qualitative test. Dako PD-L1 IHC 28-8 pharmDx.
PD-L1 IHC 22C3 is a qualitative immunohistochemical assay using Monoclonal Mouse Anti-PD-L1 Clone 22C3 intended for use in the detection of PD-L1 protein. The VENTANA PD-L1 SP142 Assay is a qualitative immunohistochemical assay using rabbit monoclonal anti-PD-L1 clone SP142 intended for use in the assessment of the PD-L1 protein in formalin-fixed paraffin-embedded FFPE triple-negative breast carcinoma TNBC tissue on a VENTANA BenchMark ULTRA instrument. The PD-L1 companion diagnostic test for Keytruda immunohistochemistry clone 22C3 is interpreted using a Tumor Proportion Score TPS.
These agents called PD-1 or PD-L1 inhibitors or checkpoint inhibitors have been shown to increase immune responses to cancers and improve patient survival. 6 rijen Order Code Order Code Name Order Loinc Result Code Result Code Name UofM Result LOINC. In 2014 the FDA granted accelerated approval for pembrolizumab Keytruda to treat advanced or unresectable melanoma making it the first PD.
For other FDA approved primary tumors please order the following. VENTANA PD-L1 SP142 Assay is the first and only FDA-approved test for TECENTRIQ in triplenegative breast cancer TNBC urothelial carcinoma UC. May 11 2018 by Linda.
Lung PD -L1 Lung Nivolumab IHC 93359 88360 FFPE. See CPB 0890 - Pembrolizumab Keytruda. Options Companion and Complementary Diagnostic Assays in Special Instructions.
Test Name Comp Test Code CPT Code Specimen Type New assays PD -L1 Lung a tezolizumab IHC 94480 88360 FFPE. PD-L1 immunohistochemistry IHC is indicated in patients with specific tumor types in order to predict their responses to treatment with PD-L1 inhibitors. PD-L1 has been identified as both a prognostic and theranostic marker in a variety of neoplasms.
Clone 22C3 88360 G0452 3-5 Tissue PD-L1 Expression by ImmunoHistoChemistry IHC. For NSCLC order 93279-PD-L1 Lung Pembrolizumab IHC 93359-PD-L1 Lung Nivolumab IHC or 94480-PD-L1 Lung Atezolizumab IHC. COMMONLY ORDERED TEST AND CPT CODE CHART updated 2182021.
Component Test Code Component Chart Name LOINC. Test Name Comp Test Code CPT Code Specimen Type PD -L1 Lung Pembrolizumab IHC 93279 88360 FFPE. Detecting PD-L1 in tumor cells identifies cancer patients that may benefit from treatment with immunotherapy agents.
G0452MLH1 Methylation use only if needed to accompany previous MSI testing 5-7 Tissue PD-L1 Expression by. For gastricGEJ adenocarcinoma urothelial carcinoma cervical carcinoma or head and neck squamous cell carcinoma HNSCC specimens see PD-L1 22C3 IHC with Combined Positive Score CPS.
 In Silico Simulation Of A Clinical Trial With Anti Ctla 4 And Anti Pd L1 Immunotherapies In Metastatic Breast Cancer Using A Systems Pharmacology Model Royal Society Open Science
In Silico Simulation Of A Clinical Trial With Anti Ctla 4 And Anti Pd L1 Immunotherapies In Metastatic Breast Cancer Using A Systems Pharmacology Model Royal Society Open Science
 Modulation Of Intratumoural Myeloid Cells The Hallmark Of The Anti Tumour Efficacy Induced By A Triple Combination Tumour Associated Peptide Tlr 3 Ligand And A Pd 1 British Journal Of Cancer
Modulation Of Intratumoural Myeloid Cells The Hallmark Of The Anti Tumour Efficacy Induced By A Triple Combination Tumour Associated Peptide Tlr 3 Ligand And A Pd 1 British Journal Of Cancer
Https Www Questdiagnostics Com Dms Documents Other Programmed Death Ligand Pd L1 Flyer Programmed Death Ligand Pdl 1 Flyer Pdf
Https Www Questdiagnostics Com Dms Documents Other Pd L1 Overview Pdf

![]() Pd L1 22c3 Fda For Nsclc Neogenomics Laboratories
Pd L1 22c3 Fda For Nsclc Neogenomics Laboratories
Https Www Questdiagnostics Com Dms Documents Other Programmed Death Ligand Pd L1 Flyer Programmed Death Ligand Pdl 1 Flyer Pdf
 Quest Diagnostics Programmed Death Ligand Pd L1 Pd L1 Programmed Death Ligand
Quest Diagnostics Programmed Death Ligand Pd L1 Pd L1 Programmed Death Ligand
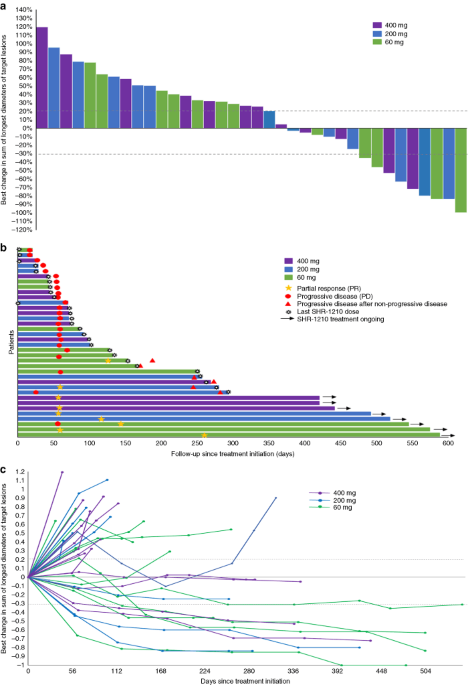 Safety Anti Tumour Activity And Pharmacokinetics Of Fixed Dose Shr 1210 An Anti Pd 1 Antibody In Advanced Solid Tumours A Dose Escalation Phase 1 Study British Journal Of Cancer
Safety Anti Tumour Activity And Pharmacokinetics Of Fixed Dose Shr 1210 An Anti Pd 1 Antibody In Advanced Solid Tumours A Dose Escalation Phase 1 Study British Journal Of Cancer


No comments:
Post a Comment
Note: Only a member of this blog may post a comment.