In most cases a hospital testing clinic or DNA laboratory can have paternity DNA test results back to you in as little as a three business days to a week. 1 Release of ctDNA into the blood allows for a.
 Longitudinal Cell Free Dna Analysis In Patients With Small Cell Lung Cancer Reveals Dynamic Insights Into Treatment Efficacy And Disease Relapse Journal Of Thoracic Oncology
Longitudinal Cell Free Dna Analysis In Patients With Small Cell Lung Cancer Reveals Dynamic Insights Into Treatment Efficacy And Disease Relapse Journal Of Thoracic Oncology
Screens for some physical defects.
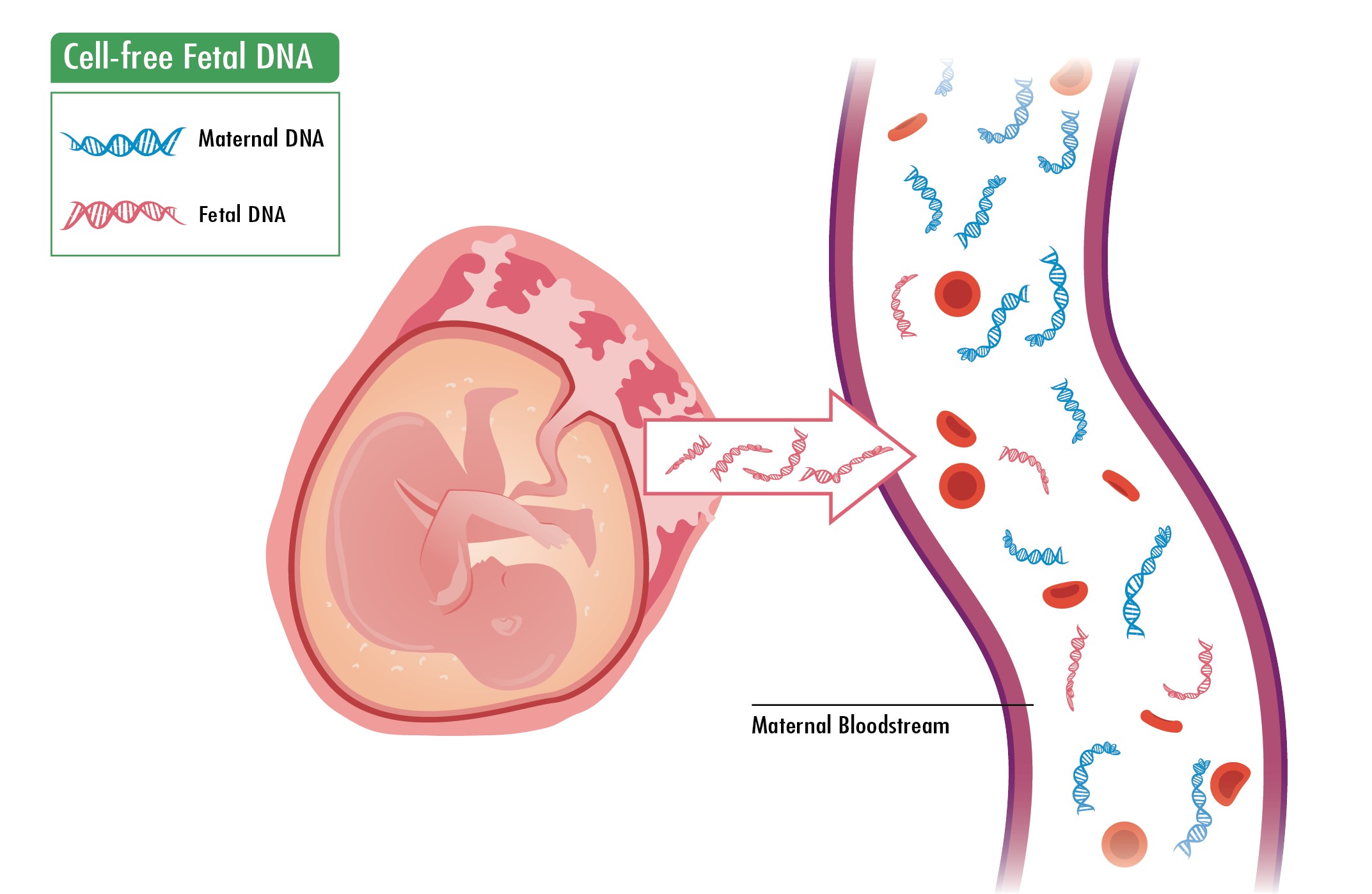
Cell free dna test timing. Some of the genetic material DNA from the pregnancy circulates in the pregnant womans blood. Two 10 mL whole blood in Streck Cell-Free DNA BCT tubes mottled black and tan. Tumour cells may release circulating cell-free tumour DNA ctDNA which may contain identical mutations to those identified in the primary tumour.
Pre-test counseling for women with obesity should include the increased chance for a test failure. The majority of women weighing less than 400 pounds will receive a test result and over 80 of women with a weight 400 pounds will receive a test. Screens for Down syndrome trisomies 13 and 18 and sex chromosome abnormalities.
Screens for Down syndrome trisomy 18 and NTDs. The recent release of new non-invasive prenatal tests for fetal aneuploidy using cell-free fetal DNA cffDNA has been hailed as a revolution in prenatal testing and has triggered significant commercial interest in the field. Test results are generally available within seven to 10 days.
Samples drawn into these tubes are stable. The Harmony Prenatal Test is a non-invasive prenatal test NIPT based on cell-free DNA analysis and is considered a prenatal screening test not a diagnostic test. Gently mix blood by inverting tube 8-10 times.
NIPT has great potential as a screening test. Second-trimester Screening quad screen Timing. The results of this calculator only apply to patients who have a result from NIPTcfDNA.
A cell-free DNA test can be done as early as 10 weeks of pregnancy and up until delivery. Pre-test counseling for women with obesity should include the increased chance for a test failure. These are advertised to prevent lysis at ambient temperature for up to two weeks but we and others have noted that processing within a week or less is more appropriate to minimize contamination 44.
Although the risk of a no call result increases with maternal weight cell-free DNA screening should be offered to all women at 9-12 weeks gestation allowing the option to have chorionic villus sampling following a positive test result. The majority of women weighing less than 400 pounds will receive a test result and over 80 of women with a weight 400 pounds will receive a test. What is cell-free DNA screening.
10 weeks and beyond. An alternative are the cell-free DNA BCT tubes from Streck. Ongoing research portends the arrival of a wide range of cffDNA tests.
With this test a sample of the womans blood is taken after 10 weeks of pregnancy. Of course anytime you send of DNA or. This calculator will allow you to estimate the Positive Predictive Value PPV and Negative Predictive Value NPV of noninvasive prenatal tests NIPT also known as Cell Free DNA Screening cfDNA based on estimates of population prevalence or by entering your own prevalence numbers.
This test is also called NIPT which stands for non-invasive prenatal test because it involves simply taking a sample of blood from the moms vein rather than inserting a needle into the uterus like an amniocentesis. Cell-free DNA is a new lab test offered during pregnancy that is used to screen for Down syndrome. However it is not yet clear how these tests will be integrated into well-established prenatal testing strategies in the USA as the timing of such testing.
CELL-FREE TUMOUR DNA TESTING IN CANCER Cell-free DNA cfDNA are small fragments of DNA that are released from normal cells and tumour cells by programmed cell death apoptosis into the blood. The test measures the small fragments of fetal DNA in. But if you choose to hold off on a DNA test until some time later or if you need a DNA test long after your child has grown out of baby clothes paternity DNA test results are still relatively fast.
Harmony does not screen for potential chromosomal or genetic conditions other than those expressly identified here. Streck Cell-Free DNA BCT Streck USA are blood collection tubes with a proprietary stabilisation buffer intended for the collection shipping and storage of whole blood samples for clinical cfDNA analysis. In this issue of the Journal Gross and colleagues present results from the first 6 months of clinical testing for 22q112 deletion syndrome 22q112 DS using a singlenucleotide polymorphism SNPbased cellfree DNA cfDNA assay 1In contrast to the numerous clinical validation studies published in rapid succession on cfDNA screening for Down syndrome data on the new practice of.
Cell-free DNA screening is a test that can determine if a woman has a higher chance of having a fetus with Down syndrome trisomy 21 trisomy 18 trisomy 13 or an abnormality in the sex chromosomes X and Y chromosomes. Although the risk of a no call result increases with maternal weight cell-free DNA screening should be offered to all women at 9-12 weeks gestation allowing the option to have chorionic villus sampling following a positive test result. How Is It Done.
All women should discuss their results with their healthcare provider who can recommend confirmatory diagnostic.